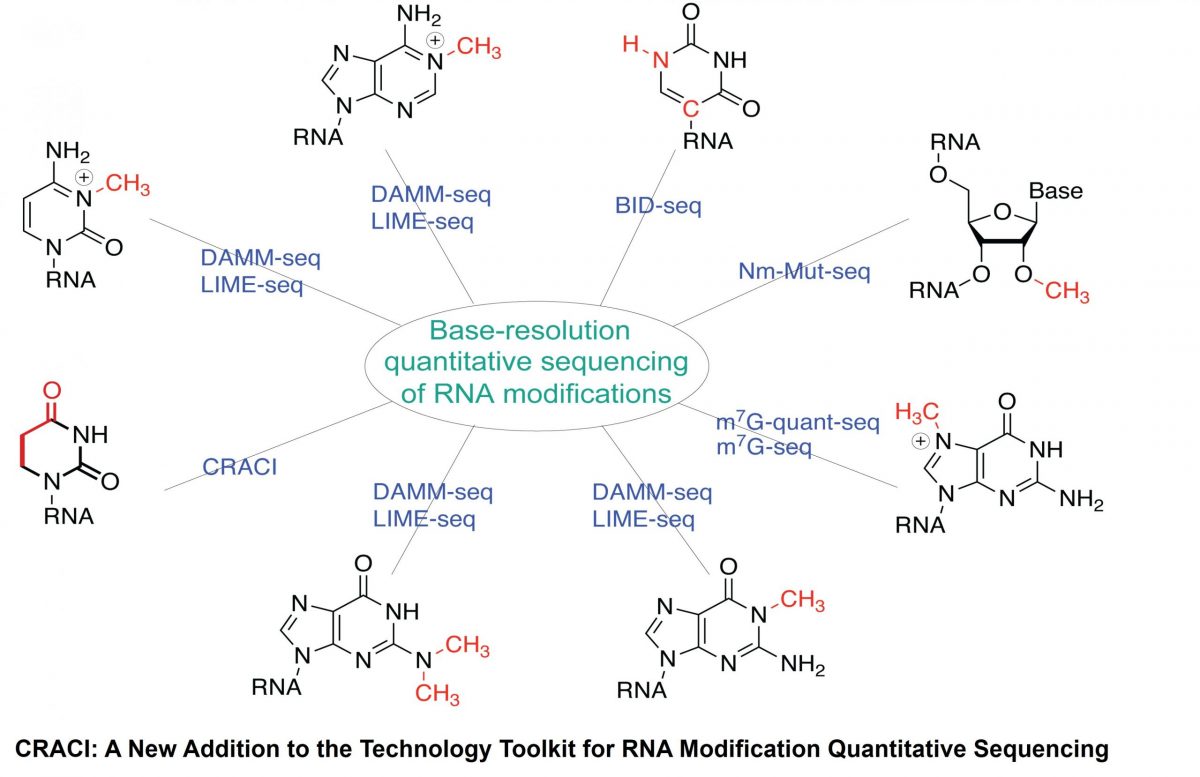
A team led by Prof. ZHANG Li‑Sheng (Division of Life Science & Department of Chemistry, HKUST) has developed Chemical Reduction Assisted Cytosine Incorporation sequencing (CRACI) — a sensitive method that quantitatively pinpoints dihydrouridine (D), the most abundant tRNA modification at the single-base resolution.
Small chemical marks on RNA—like D—can work like fine‑tuning structural switches, influencing how genes are expressed. Until now, these D marks were hard to detect. With CRACI, the team created the first detailed maps of D in cellular tRNAs from mammals and plants, including those inside mitochondria.
Key discoveries:
Because D is common and conserved across species, and related enzymes are potentially linked to human disease, CRACI gives researchers a powerful platform to explore how these RNA marks shape cell function, stress responses, and health. The team plans to extend CRACI to primary cells and tissues to build a clear and comprehensive picture of D roles in disease contexts.
Journal reference
Ju, CW., Li, H., Jiang, B. et al. Quantitative CRACI reveals transcriptome-wide distribution of RNA dihydrouridine at base resolution. Nat Commun 16, 8863 (2025). https://doi.org/10.1038/s41467-025-63918-w
Read More
Prof. ZHAI Yuanliang, Associate Professor in the Division of Life Science (LIFS), has been awarded the Collaborative Research Project Grant (CRPG) under the Collaborative Research Fund (CRF) 2025/26. He was awarded HKD7.96 million for the project “Molecular Mechanisms of Replisome Coupling” in collaboration with The University of Hong Kong (HKU).
Read More
After four years of systematic investigation, Prof. Dang’s team bypassed the need for purification with detergents by directly generating vesicles containing the target protein from cell membranes. This approach produced samples suitable for cryo-EM imaging and structural studies. The team established a comprehensive workflow for the preparation, purification, and quality control of vesicle samples, making this method applicable to various membrane systems. To address the strong background signal and interference caused by the native membrane structure, they developed a micrograph-based sorting approach integrated with an artificial intelligence model to specifically isolate high-quality membrane protein particles. They successfully applied this method to multiple membrane protein systems, resolving the structure of the overexpressed AcrB protein in E. coli cell membranes at 3.9 Å resolution and the structure of the native respiratory chain complex III in porcine heart mitochondrial inner membranes at 3.0 Å resolution.
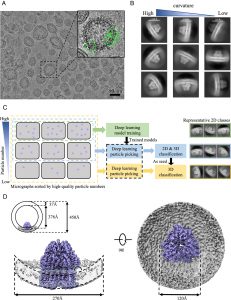
A scientific picture of Cryo-EM structure determination of AcrB in vesicle.
LIU Hang, a Ph.D. candidate from Prof. Dang’s team and the first author of the study, reflected: “Leveraging Prof. Dang’s multidisciplinary approach, our team has successfully developed a comprehensive system for in-situ structural studies of membrane proteins, encompassing both sample preparation and data processing, which overcomes challenges that were previously insurmountable. “
Compared to traditional detergent-based methods, this novel approach offers key advantages, including lower cost, simpler operation, and greater ease of use. Crucially, it preserves the native membrane environment and key lipid molecules, maintaining the protein’s natural conformation to the greatest extent possible. The method also demonstrates versatility, making it applicable to different membrane proteins across various species and cellular membrane structures. It promises to significantly reduce the workload for researchers, simplify the determination of membrane protein structures, and broaden the scope of cryo-EM structural biology.
“This vesicle-based platform preserves physiological lipid environments while eliminating the burdens of detergent screening,” explained Prof. Dang. “It provides an opportunity to study membrane proteins in their native environments. With further optimization, we aim to achieve structural proteomics of membrane proteins in specific biological membrane systems, such as mitochondria, under various physiological and pathological conditions, offering valuable insights into diseases.”
This research was published in the Proceedings of the National Academy of Sciences (PNAS). Prof. DANG Shangyu is the corresponding author, Ph.D. candidate LIU Hang is the first author, and undergraduate student TSE Chun Mong is the second author.
Repost from
Read More
The secretory pathway in eukaryotic cells is crucial for maintaining cellular function and physiological activities, as it ensures the accurate transport of proteins to specific subcellular locations or for secretion outside the cell. A research team led by Prof. GUO Yusong from the Division of Life Science at the Hong Kong University of Science and Technology (HKUST) has been extensively investigating the molecular mechanisms by which cargo proteins are recognized and loaded into transport vesicles in the secretory pathway. The team has successfully reconstituted the packaging of multiple disease-related cargo proteins into vesicles along the secretory route, providing a powerful tool for dissecting the molecular mechanisms of cargo loading. In addition, they developed an innovative analysis platform that integrates vesicle reconstitution with electron microscopy and proteomics, enabling systematic identification of vesicle protein composition and morphological features. This comprehensive approach has proven effective in uncovering novel cargo clients and cellular factors that mediate vesicular trafficking (Figure 1).
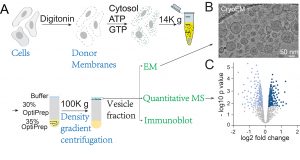
Figure 1: Schematic of the research approach based on vesicle reconstitution combined with electron microscopy and proteomics. (References: PNAS 2019, PNAS 2021, PNAS 2025, Nature Plants 2021)
The research team led by Prof. Guo, in collaboration with Prof. YAO Zhong-Ping’s team at The Hong Kong Polytechnic University (PolyU), published their findings in PNAS. This study integrates in vitro vesicle reconstitution with quantitative mass spectrometry to systematically identify transport cargos mediated by the adaptor protein complexes AP-1 and AP-4. It also elucidates key cytosolic regulatory factors involved in AP-4–mediated vesicle trafficking.
Within the secretory pathway, the trans-Golgi network (TGN) serves as a central sorting hub, packaging proteins precisely into transport vesicles. Sorting errors can prevent cargos from reaching their correct targets, leading to defects in cell polarity, immune function, and other physiological processes. Key factors involved in protein sorting at the TGN are adaptor protein (AP) complexes, AP-1 and AP-4. Mutations in their genes are closely linked to several human diseases: AP1S1 mutations (encoding the σ1 subunit of AP-1) are associated with MEDNIK syndrome; AP1S2 mutations can cause X-linked intellectual disability; and mutations in any AP-4 subunit lead to “AP-4 deficiency syndrome,” a complex hereditary spastic paraplegia. Therefore, defining the cargo clients of AP-1 and AP-4 is crucial for understanding their physiological and pathological roles. However, the full repertoire of cargos and cofactors they mediate remains unclear. Notably, AP-4–mediated TGN export appears to be clathrin-independent, suggesting that vesicle formation may depend on yet-unidentified accessory factors.
In this study, Prof. Guo’s team performed vesicle formation assays using AP1γ1 or AP4ε knockout cells, isolated vesicle fractions, and conducted proteome-wide analysis by quantitative mass spectrometry. This approach allowed them to identify AP-1– and AP-4–dependent cargo proteins exported from the TGN, as well as key cytosolic accessory factors required for AP-4–mediated transport. Subsequent biochemical validation revealed that CAB45 is an AP-1–dependent cargo, while ATRAP (an angiotensin II type I receptor–associated protein) is an AP-4–dependent cargo. Notably, AP-4 recognizes a tyrosine-based motif at the cytosolic terminus of ATRAP to mediate its loading into transport vesicles from the Golgi.
The study also found that the cytosolic proteins WDR44 and PRRC1 play critical roles in AP-4–mediated cargo sorting. When WDR44 levels were knocked down, the AP-4 cargo ATG9A abnormally accumulated at the Golgi. Similarly, PRRC1 knockout caused ATG9A to be retained in the endoplasmic reticulum, impairing cellular autophagy. Another AP-4 cargo, ATRAP, also accumulated in the Golgi under these conditions. “These results not only deepen our understanding of AP-1 and AP-4 functions in secretory trafficking but also provide a powerful methodological toolkit for systematically dissecting the mechanisms of specific accessory factors,” said Prof. Guo.
The study’s co-corresponding authors are Prof. Guo of HKUST and Prof. Yao of PolyU. Dr. PENG Ziqing, a postdoctoral researcher at HKUST, is the first author.
Repost from
Read More
A landmark study conducted by The Hong Kong University of Science and Technology (HKUST) has demonstrated that a genetic variant, TREM2 H157Y, significantly increases the risk of Alzheimer’s disease (AD) in individuals of ethnic Chinese descent. The research reveals that this variant confers a risk level comparable to that of the strongest known genetic risk factor for AD, APOE-ε4, and is associated with more rapid clinical progression and more severe neurodegeneration.
This is the first in the field to conduct an in-depth family-based clinical case study on a Chinese-enriched genetic risk factor for AD, the TREM2 H157Y variant. The findings, published in Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, have profound implications for disease monitoring and patient management.
Highlights of the Findings
AD currently affects approximately 10 million people in Chinese Mainland, with the number projected to rise to around 50 million by 2050. Genetic factors account for 60-80% of the risk for late-onset AD, with the APOE gene being the most extensively studied, followed by TREM2. Both genetic risk factors exhibit significant differences in prevalence and risk effects across different ethnic populations. In particular, the most studied TREM2 genetic variant, R47H, is enriched in European populations but not found in Chinese populations. As most AD genetic studies have been conducted in European populations, it is crucial to undertake such studies on diverse populations.
To address this gap, the Hong Kong Center for Neurodegenerative Diseases (HKCeND) launched its Biobank (HKCeND Biobank) for AD research. Led by HKUST and in collaboration with hospitals in Hong Kong, this biobank collects and consolidates comprehensive clinical, neuroimaging, and multi-omics data from individuals of ethnic Chinese origin. This makes it a critical resource for investigating Chinese-enriched genetic factors such as the TREM2 H157Y variant.
This research on the genetic variant was conducted by a team led by Prof. Nancy IP, President and the Morningside Professor of Life Science at HKUST. She is also the Director of the State Key Laboratory of Nervous System Disorders (SKLNSD) at HKUST and the Center Director of the InnoHK HKCeND.
The study resulted in several novel findings. The clinical case study showed that TREM2 H157Y variant carriers with the APOE-ε4 risk factor exhibit more rapid clinical progression in the early stages of the disease, compared to non-carriers. Cognitive assessments, neuroimaging, and AD blood biomarker analyses indicated that AD patients carrying the TREM2 H157Y variant experience significantly worsened cognitive functions, more severe neurodegeneration, and more severe AD pathology. Additionally, blood protein profile analysis revealed alterations in immune, vascular, and bone-related biological processes in these individuals. This highlights the use of blood biomarkers to investigate how genetic risk factors influence AD pathology, generating new insights into AD biology and novel therapeutic development.
Prof. Ip remarked, “This study is the first to demonstrate that the TREM2 H157Y genetic variant is associated with more severe AD pathology and neurodegeneration. The prevalence of the TREM2 H157Y variant among ethnic Chinese individuals emphasizes the importance of conducting genetic studies in the Chinese population.” She added, “This study also highlights the critical clinical implications of the genetic variant for timely intervention and personalized disease management. Our research benefits from public participation and close collaboration between clinicians and scientists, effectively linking scientific findings to improved patient outcomes.”
The study was supported by multiple funding bodies, including the InnoHK initiative of the Innovation and Technology Commission (ITC) of the Hong Kong Special Administrative Region Government, the Areas of Excellence Scheme of the University Grants Committee, the Research Grants Council (RGC) of Hong Kong, the ITC Grant, the Chow Tai Fook Charity Foundation, the National Natural Science Foundation of China (NSFC)/RGC Joint Research Scheme, and the SIAT-HKUST Joint Laboratory for Brain Science, under the Chinese Academy of Sciences. The clinical case study was conducted in collaboration with Prof. Timothy Kwok from the Prince of Wales Hospital, The Chinese University of Hong Kong.
Repost from
Read More
Diffuse glioma is a type of brain tumor that can vary significantly in behavior and response to treatment. Among them, isocitrate Dehydrogenase (IDH)-mutant astrocytoma represents a major subtype. These tumors are particularly aggressive and often recur after treatment. Understanding the different molecular subtypes of these tumors is crucial for improving patient management.
In a recent study led by Prof. Jiguang WANG from the Division of Life Science (LIFS) and Department of Chemical and Biological Engineering (CBE), along with his students Jihong TANG (LIFS) and Yuyan RUAN (CBE), in collaboration with colleagues from Beijing Tiantan Hospital and Shanghai Huashan Hospital, researchers identified four major proteomics subtypes, and found out that the Immune/Mesenchymal-Enriched (IME) subtype accounts for about 13% of cases. This subtype is characterized by unique immune features, specific gemistocytic differentiation, and poorer survival rates. Notably, the IME subtype tends to become more common in recurrent tumors.
Key Findings
Researchers identified four proteomics subtypes of IDH-mutant astrocytomas:
Highlights
The team integrated multi-omics, single-cell sequencing, and spatial omics with an AI-powered analytical framework to gain insights into the tumor’s biology. This approach revealed that certain genes related to immune response are highly active in IME tumors, potentially explaining their aggressive nature.
This work opens new avenues for understanding and treating these tumors. An AI-powered tool was also developed to help classify patients, paving the way for more personalized treatment strategies. The researchers are now undertaking deeper mechanistic studies and exploring therapeutic strategies for these patients. Overall, this research enhances our understanding of IDH-mutant astrocytomas and aims to improve outcomes for patients facing this challenging disease.
Publication
JihongTang, Wenhua Fan, Yuyan Ruan, Xing Liu, Fufang Qiu, Jie Feng, Guoshi Huang, Mengli Yan, Hui Wang, Quanhua Mu, Ran Liu, Yingxi Yang, Zhi Huang, Yimeng Qiao, Xuejie Wang, YumengGuo, Mingchen Yu, Ying Zhang, Ruichao Chai, Fan Wu, Zheng Zhao, Zhaoshi Bao, Wei Hua, Kai Liu, Qianghu Wang, Ying Mao, Qing Chang, Tao Jiang, Jiguang Wang. Protein-based classification reveals an immune-hot subtype in IDH mutant astrocytoma with worse prognosis. Cancer Cell, 2025, DOI: 10.1016/j.ccell.2025.08.006.
Read More
Prof. Angela WU, Associate Professor in the Division of Life Science and the Department of Chemical and Biological Engineering at the Hong Kong University of Science and Technology (HKUST), has been named a 2025 Schmidt Polymath, a prestigious recognition celebrating transformative research across disciplines.
This year, eight outstanding scientists and engineers from universities worldwide have been honored as Schmidt Polymaths. Organized by Schmidt Sciences, a philanthropic organization founded in 2024, the program aims to accelerate scientific knowledge and breakthroughs by supporting researchers with advanced tools and resources. Each awardee will receive up to USD $2.5 million over five years to pursue bold, high-impact research projects that push conventional boundaries.
Prof. Wu earned this recognition through a rigorous global selection process. As a bioengineer, she is recognized for her pioneering work at the intersection of biology and engineering. With the support of the Schmidt Polymaths program, Prof. Wu will continue her innovative research to further develop fully human, functional in-vitro brain organoids, with the ultimate goal of enabling therapeutic transplantation.
Since joining HKUST in 2015, Prof. Wu has made significant contributions in the fields of single-cell genomics, computational biology, and microfluidics. Her groundbreaking achievements have earned her numerous accolades, including being named to the MIT Technology Review’s “Innovators Under 35 Asia Pacific” list in 2017, selected as a World Economic Forum Young Scientist in 2018, and receiving the Excellent Young Scientists Fund from the National Natural Science Foundation of China in 2024.
Source
https://hkust.edu.hk/news/hkust-bioengineer-prof-angela-wu-named-2025-schmidt-polymath

Although researchers usually rely on mice for studies, grey mouse lemurs being primates, are closer to us on the evolutionary tree and provide insights into biology that truly matter to humans.
Prof. Angela WU, associate professor in the Division of Life Science and Department of Chemical and Biological Engineering, collaborated with other researchers to map over 226,000 single cells from 27 organs of the grey mouse lemur. This groundbreaking work uncovered hundreds of cell types and key genes that mice simply don’t have, reshaping how we study human biology and disease.
These pioneering studies have recently been published in two papers in Nature, marking a significant advancement in the field.
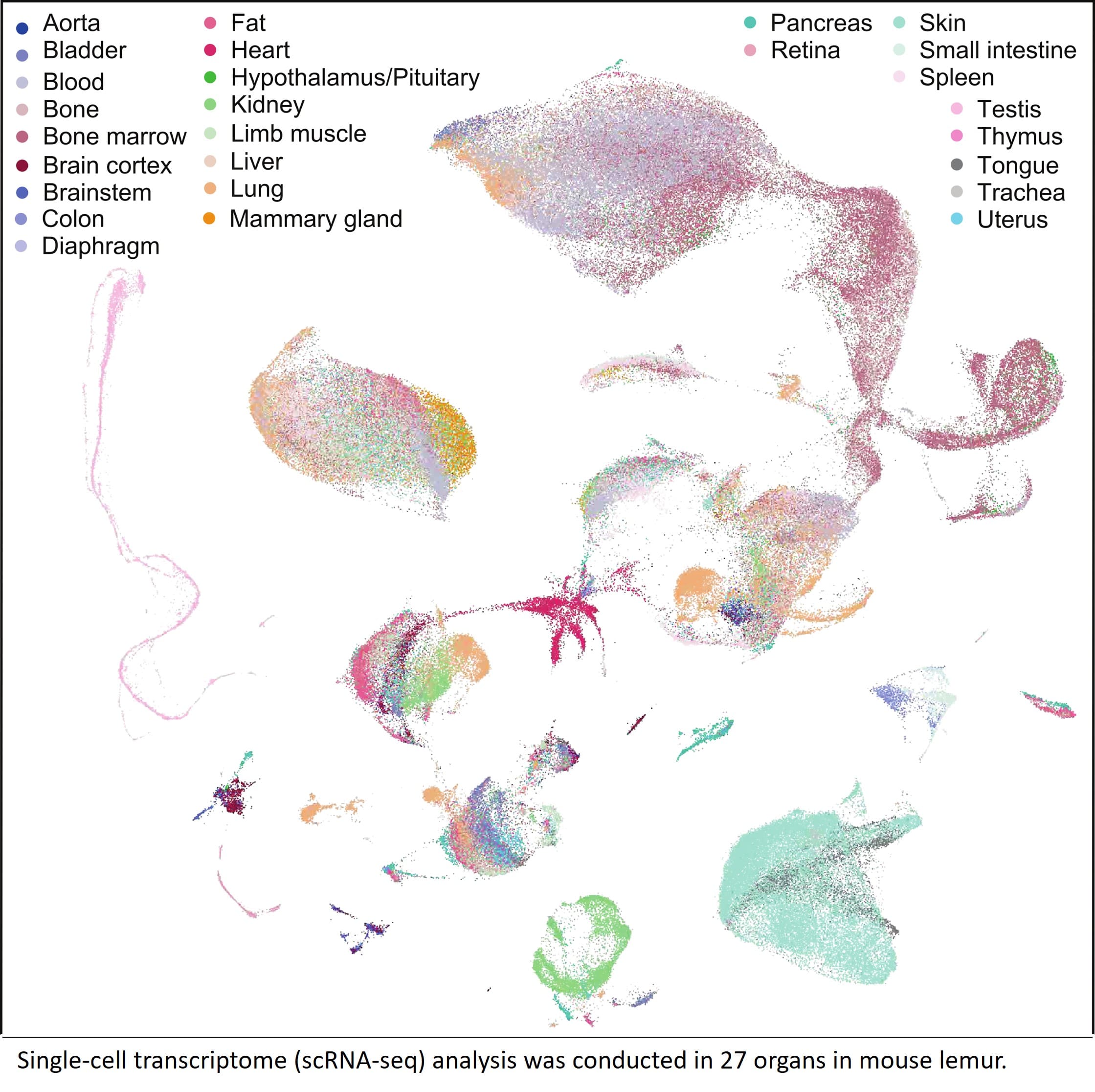
Publications
Ezran, C., Liu, S., Chang, S. et al. Mouse lemur cell atlas informs primate genes, physiology and disease. Nature (2025). https://doi.org/10.1038/s41586-025-09114-8
The Tabula Microcebus Consortium., Ezran, C., Liu, S. et al. A molecular cell atlas of mouse lemur, an emerging model primate. Nature (2025). https://doi.org/10.1038/s41586-025-09113-9
Professor Li-Sheng ZHANG, (Division of Life Science and Department of Chemistry, HKUST) and his research team have recently made a significant breakthrough in cancer detection by introducing a new RNA modification method called LIME-seq (Low-Input Multiple Methylation Sequencing) which has been published in Nature Biotechnology. This innovative approach focuses on analyzing circulating free RNA (cfRNA) in the blood, which has the potential to improve early cancer diagnosis, particularly for challenging cases like colorectal cancer.
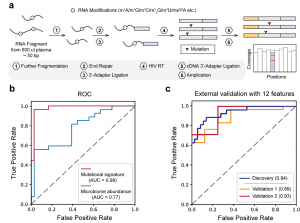
Overview of the LIME-seq workflow. b, Receiver operating characteristic (ROC) curves support the superior performance of mutation signatures compared to microbiome abundance obtained from LIME-seq. c, ROC curves showing that 12 microbiome-derived biomarkers on cfRNA distinguish patients with CRC from noncancer-bearing controls across discovery (that is, training) and two validation cohorts.
Liquid biopsy technology has become a key method for diagnosing and monitoring cancer. It involves testing for biomarkers in the blood, such as circulating free DNA (cfDNA) and cfRNA. While cfDNA testing has gained traction, it often struggles to detect early-stage cancers like colorectal cancer (CRC) due to the low levels of cfDNA present. This has prompted scientists to explore cfRNA as a promising alternative.
cfRNA consists of tiny RNA fragments found in the blood, which are released from dying cells or tissue turnover. Although cfRNA has historically been difficult to work with due to its fragility and low quantity, it offers unique advantages. It provides insights into gene activity and can signal early changes related to cancer, making it potentially more useful than traditional DNA tests.
LIME-seq stands out for its ability to detect RNA modifications with minimal sample input—requiring less than 2 ng of RNA. The researchers demonstrated that this method could identify various RNA changes linked to cancer. In their studies, they analyzed plasma samples from colorectal cancer patients and found elevated levels of microbial-derived cfRNA methylation, leading to the development of a highly accurate model for distinguishing cancer patients from healthy individuals.
LIME-seq has three major highlights:
The findings from this research highlight how cfRNA modifications could be used as non-invasive tools for early cancer detection. The connection between microbial cfRNA and tumor status opens new possibilities for understanding how the human microbiome interacts with cancer. This innovative approach not only promises advancements in cancer diagnostics but also paves the way for exploring other diseases, making it a significant step forward in medical research and patient care.
Publication reference
Ju, CW., Lyu, R., Li, H. et al. Modifications of microbiome-derived cell-free RNA in plasma discriminates colorectal cancer samples. Nat Biotechnol (2025). https://doi.org/10.1038/s41587-025-02731-8

The project titled “Neuroimmune Mechanisms and Modulation in Alzheimer’s Disease led by Professor BU Guojun, Head of the Division of Life Science, has been awarded the Research Grant’s Council Theme-based Research Scheme (TRS) Award (2025/26).
Project Details
This project investigates how our brain and body’s immune systems affect Alzheimer’s disease. Leveraging cutting-edge technologies, researchers aim to differentiate protective and harmful immune responses. This knowledge will guide the development of targeted treatments to benefit patients, families, and society, while supporting Hong Kong’s healthy aging initiatives and economic growth. The project has been granted an approved budget of $76.206 million.
For more details please visit https://hkust.edu.hk/news/hkust-researchers-dominate-rgc-funding-awards-record-breaking-success.