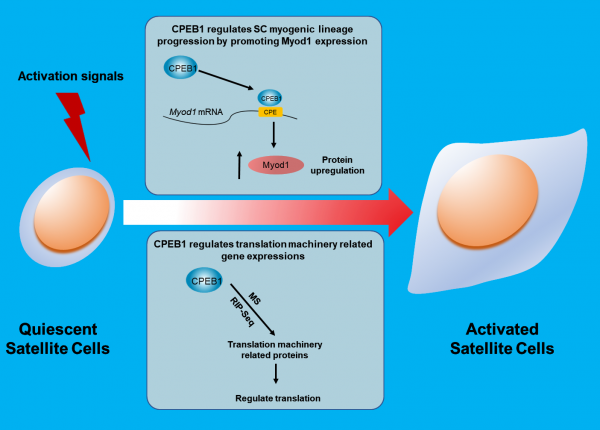
In this study published in Nature Communications, Prof. Tom Cheung and his team identified cytoplasmic polyadenylation elements-binding protein 1 (CPEB1) as a critical regulator in reprogramming the translational landscape upon SC activation.
Read More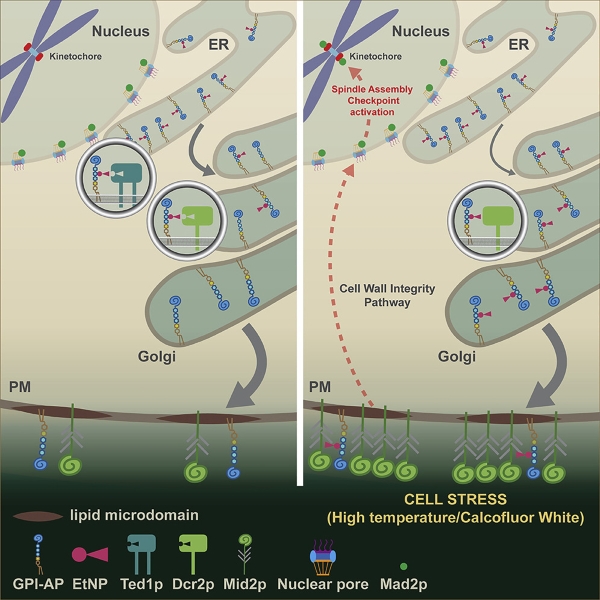
In the current study published in Cell Reports, Prof. David Banfield and his research team have discovered two remodeling events crucial in yeast cell survival.
Read More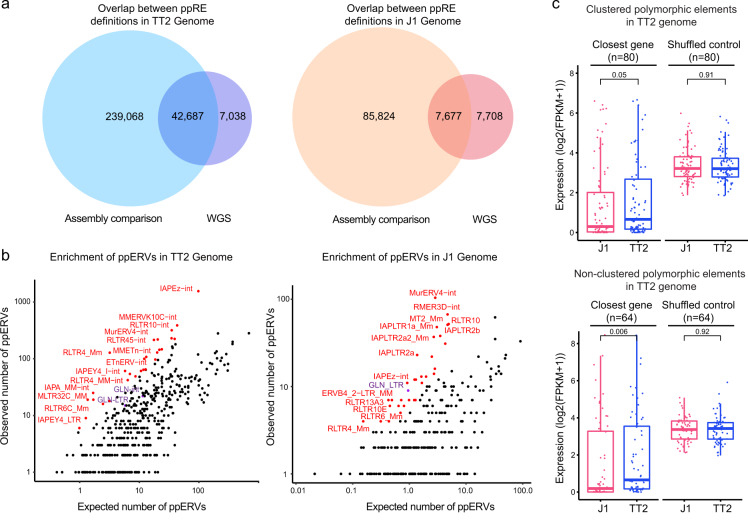
Prof. Danny Leung and his team identified thousands of potential polymorphic endogenous retroviruses, shedding light on how the relics of viruses can shape our epigenome and give rise to different phenotypes between individuals.
Read More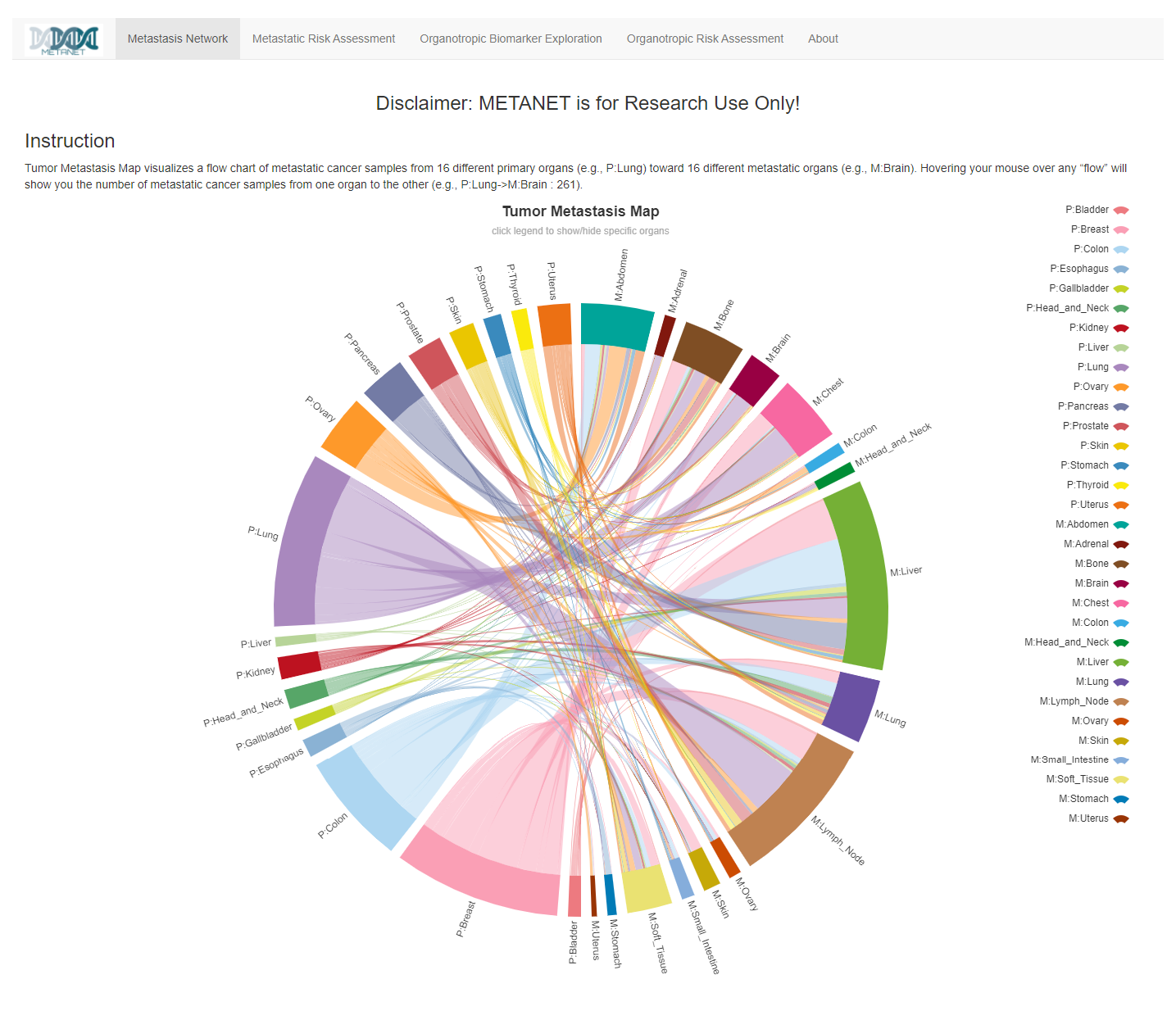
Prof. Jiguang Wang and his team developed a computational framework (termed MetaNet) that integrates clinical and sequencing data from 30K cancers to assess the metastatic risk of primary tumors.
Read More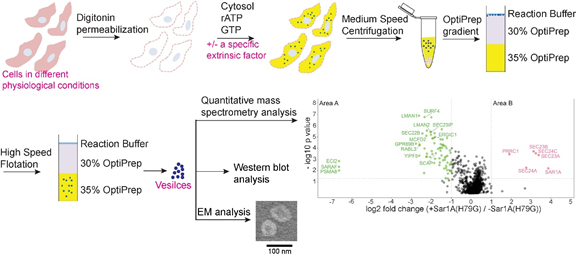
Prof. Yusong Guo and his collaborator, Prof. Zhongping Yao, at Hong Kong Polytechnic University developed an in vitro vesicle formation assay to reveal novel cargo clients and factors that mediate vesicular trafficking, providing a robust tool to reveal novel insights into the secretory pathway.
The eukaryotic secretory pathway regulates the secretion of various growth factors, hormones and other important molecules. The fidelity of the secretory pathway depends on the accurate sorting of specific cargo proteins into transport vesicles. Defects in cargo sorting induce defects in establishing cell polarity, immunity, and other physiological processes.
The key players that mediate protein sorting in the secretory pathway include small GTPases of the Arf family and cargo adaptors. The Arf family GTPases cycle between a GDP-bound inactive state and a GTP-bound active state. Upon GTP binding, Arf proteins mediate membrane recruitment of various cytosolic cargo adaptors. Once recruited onto the membranes, these cargo adaptors recognize sorting motifs on the cargo proteins to package cargo proteins into vesicles.
Although significant progress has been achieved in understanding the general steps of cargo sorting, the spectrum of cargo clients of a specific Arf family member or cargo adaptor remains largely underinvestigated. Furthermore, since distinct cytosolic proteins are recruited to membranes by different GTP-bound Arf family proteins, systematic approaches are needed to characterize vesicle budding events associated with a specific GTP-bound Arf family protein.
In this study, the Guo lab used an in vitro assay that reconstitutes the packaging of human cargo proteins into vesicles to quantify cargo capture. Quantitative mass spectrometry analyses of the isolated vesicles revealed cytosolic proteins associated with vesicle membranes in a GTP-dependent manner. One of them, FAM84B, interacts with cargo adaptors and regulates the transport of transmembrane cargo proteins. In addition, they uncovered novel cargo proteins that depend on GTP hydrolysis to be captured into vesicles.
Next, they utilized this assay and identified cytosolic proteins that depend on a specific Arf family protein, SAR1A, to be recruited to vesicle membranes. One of the cytosolic proteins, PRRC1, is recruited to endoplasmic reticulum (ER) exit sites, interacts with the inner COPII coat. Its absence increases membrane association of COPII and affects ER-to-Golgi trafficking.
Utilizing this assay, they also identified clients of COPII vesicles. These clients include two cargo receptors, SURF4 and ERGIC53. Through analyzing the protein composition of vesicles isolated from control cells or cells depleted of SURF4 or ERGIC53, they revealed specific clients of each of these two cargo receptors.
These results indicate that the vesicle formation assay in combination with quantitative mass spectrometry analysis is a robust and powerful tool to systematically reveal cargo proteins that depend on a specific factor to be packaged into vesicles, to analyze protein profiling of transport vesicles under different physiological conditions, and to uncover cytosolic proteins that interact with a specific factor on vesicle membranes.
This study was recently published in PNAS. The research in Prof. Yusong Guo’s lab focuses on investigating molecular mechanisms regulating protein sorting in the secretory pathway. Dr. Huang Yan (a postdoctoral research associate from Prof. Guo’s lab at HKUST), Dr. Haidi Yin from PolyU and Dr. Baiying Li from CUHK are co-first authors. In addition, Mr. Yang Liu, Dr. Xiao Tang, Miss Wo Wang and Miss Zhixiao Wu from the Guo lab participated in this study.
Journal Reference:
Huang Y, Yin H, Li B, Wu Q, Liu Y, Poljak K, Maldutyte J, Tang X, Wang M, Wu Z, Miller EA, Jiang L, Yao ZP, Guo Y. An in vitro vesicle formation assay reveals cargo clients and factors that mediate vesicular trafficking. Proc Natl Acad Sci U S A. 2021 Aug 31;118(35):e2101287118. doi: 10.1073/pnas.2101287118.
Read More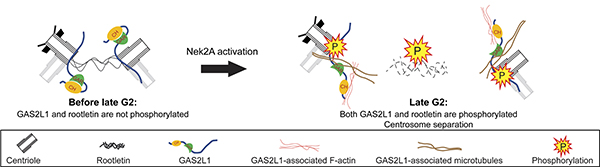
A research team led by Prof. Robert Qi revealed the mechanism of centrosome disjunction mediated by GAS2L1 during cell division.
Read More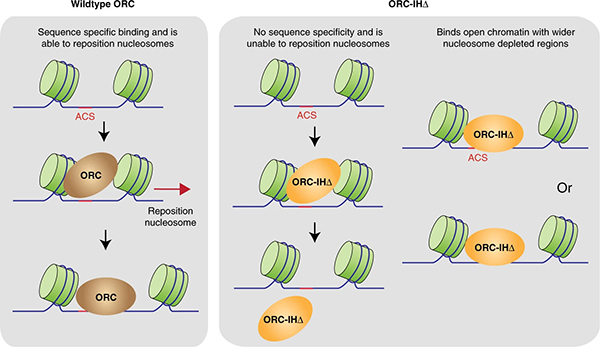
Prof. Bik-Kwoon Tye and Prof. Danny Leung, in collaboration with the University of Hong Kong (HKU), recently made a breakthrough on how the ORC selects the site for origins of replications and the basis behind the divergence between humans and yeast.
Read More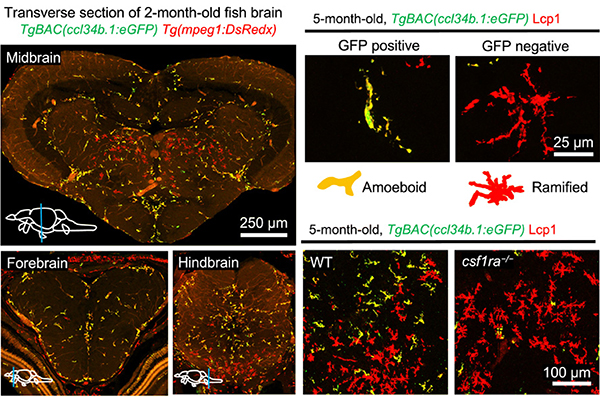
Using zebrafish as an animal model, Prof. Zilong Wen and his research team identified two distinct microglial populations – the phagocytotic microglia and regulatory microglia. Their findings were published in the journal Science Advances.
Read More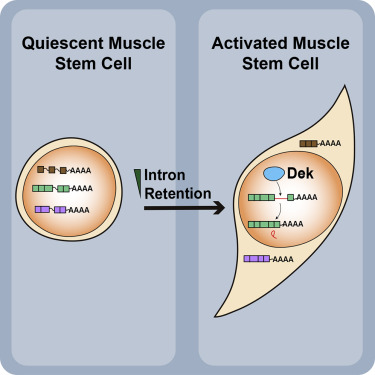
A research team led by Prof. Tom CHEUNG, Associate Professor in the Division of Life Science at HKUST, revealed the relationship between Dek and Intron retention during muscle stem cell quiescence.
Read MoreIn this study published in the journal PNAS, Prof. Tom Cheung and his research team have found that a lncRNA, LncMyoD, regulates the differentiation of muscle cells through modulating chromatin accessibility.
Read More