

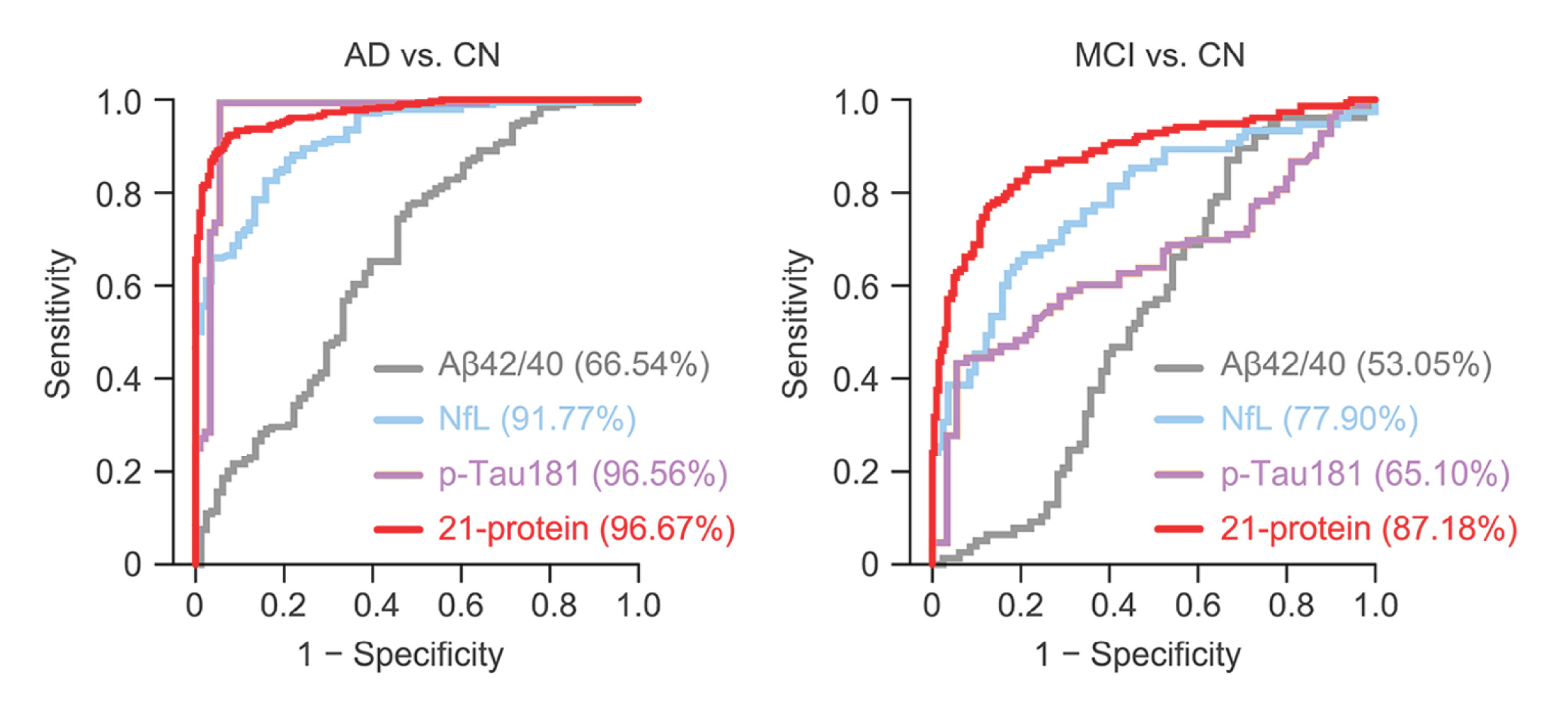
An international research collaboration led by Professor Nancy IP, has made a significant breakthrough in Alzheimer’s disease (AD) diagnosis and management. The team has developed a cutting-edge blood test for the early detection of AD and mild cognitive impairment (MCI) with remarkable accuracy rates of over 96% and 87% respectively. This blood test is applicable across diverse ethnic populations, providing a global solution to the diagnosis and management of AD.
AD is a neurodegenerative disease that affects over 50 million people worldwide. The accumulation of toxic amyloid beta (Aβ) in the brain is a major hallmark of the disease, leading to the loss of brain cells and cognitive decline. The recently approved AD drug Lecanemab targets elevated Aβ in the brain, offering new hope for treatment. However, the majority of individuals with AD and MCI go undiagnosed and untreated due to the challenges of early diagnosis. Currently, elevated Aβ can only be measured through costly brain imaging or invasive procedures, and symptoms typically appear late in the disease’s progression.
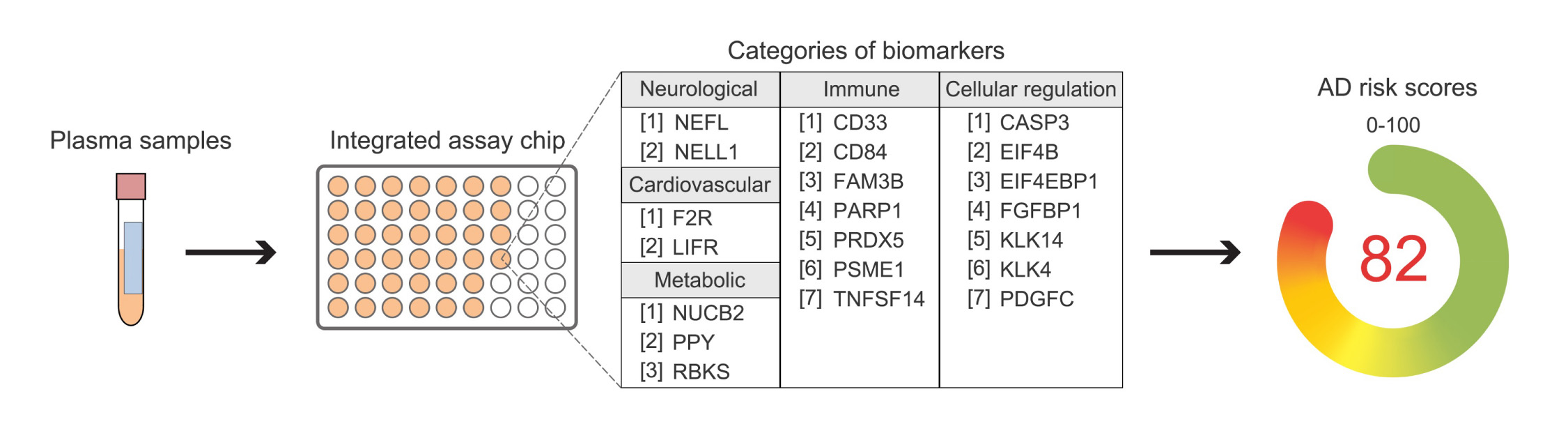
The HKUST-led research team has developed a simple blood test that accurately identifies individuals with MCI and mild AD, while also detecting elevated Aβ in the brain. The test measures the levels of 21 proteins in multiple biological pathways, providing a comprehensive profile of AD for each individual. This allows for more accurate classification of AD and MCI, as well as close monitoring of disease progression. The test has demonstrated robust performance in distinguishing individuals with AD and MCI from cognitively normal people in a multinational study involving individuals of Chinese and European descent.
The HKUST blood test represents a significant advancement in AD diagnosis and precision treatment. It offers a less invasive and more effective diagnostic method and has the potential to revolutionize the field of AD management. The test can also be used to screen individuals for suitable drug treatments in clinical studies and monitor disease progression and drug responses. Additionally, it may facilitate the development of personalized treatments by uncovering the molecular underpinnings of AD that vary between individuals and ethnicities.
The research collaboration involved University College London and the Barcelona βeta Brain Research Center, as well as clinicians from local and overseas hospitals.
The findings have been published in Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, and the technology has been licensed to a start-up company of HKUST, Cognitact Limited, to provide testing services to the public.
Journal Reference:
Jiang Y, Zhou X, Ip FC, Chan P, Chen Y, Lai NCH, Cheung K, Lo RMN, Tong EPS, Wong BWY, Chan ALT, Mok VCT, Kwok TCY, Mok KY, Hardy J, Zetterberg H, Fu AKY, Ip NY. Large-scale plasma proteomic profiling identifies a high-performance biomarker panel for Alzheimer’s disease screening and staging. Alzheimers Dement. 2022 Jan;18(1):88-102. doi: 10.1002/alz.12369.
For further information please visit: