
A joint study led by HKUST, HKU, and other institutions has been selected as one of the Top 10 Scientific Advances in China for 2023, making it the only research project from Hong Kong to be included in the list.
The study, focused on DNA replication initiation, unveiled a new mechanism in the regulation of DNA replication and was led by Prof. ZHAI Yuanliang from HKU School of Biological Sciences, Prof. DANG Shangyu from HKUST Division of Life Science (LIFS), and Prof. TYE Bik-Kwoon from HKUST Institute for Advanced Study (IAS). The team’s groundbreaking discovery of the human pre-replication complex (Pre-RC) and its atomic resolution structure provides crucial insights for developing innovative anticancer strategies that can selectively target cancer cells. The recognition received highlights the respect and esteem their work holds within the scientific community. Prof. ZHAI expressed the significance of the research in deepening our understanding of DNA replication and its potential implications in diseases like cancer. Prof. DANG emphasized the importance of their research breakthrough in enhancing the specificity of chemotherapy drugs and expressed gratitude for the support provided by the Lo Kwee Seong (LKS) Foundation in establishing the HKUST Biological Cryo-EM Center, which facilitated numerous breakthroughs in their research. The team looks forward to further advancements and hopes for new possibilities in cancer treatment.
Journal Reference:
Li, J., Dong, J., Wang, W., Yu, D., Fan, X., Hui, Y. C., Lee, C. S. K., Lam, W. H., Alary, N., Yang, Y., Zhang, Y., Zhao, Q., Chen, C., Tye, B.-K., Dang, S., Zhai, Y. The human pre-replication complex is an open complex. Cell 2023 Jan 5, 186 (1): 98-111.e21. doi.org/10.1016/j.cell.2022.12.008
Read more by clicking the link below:


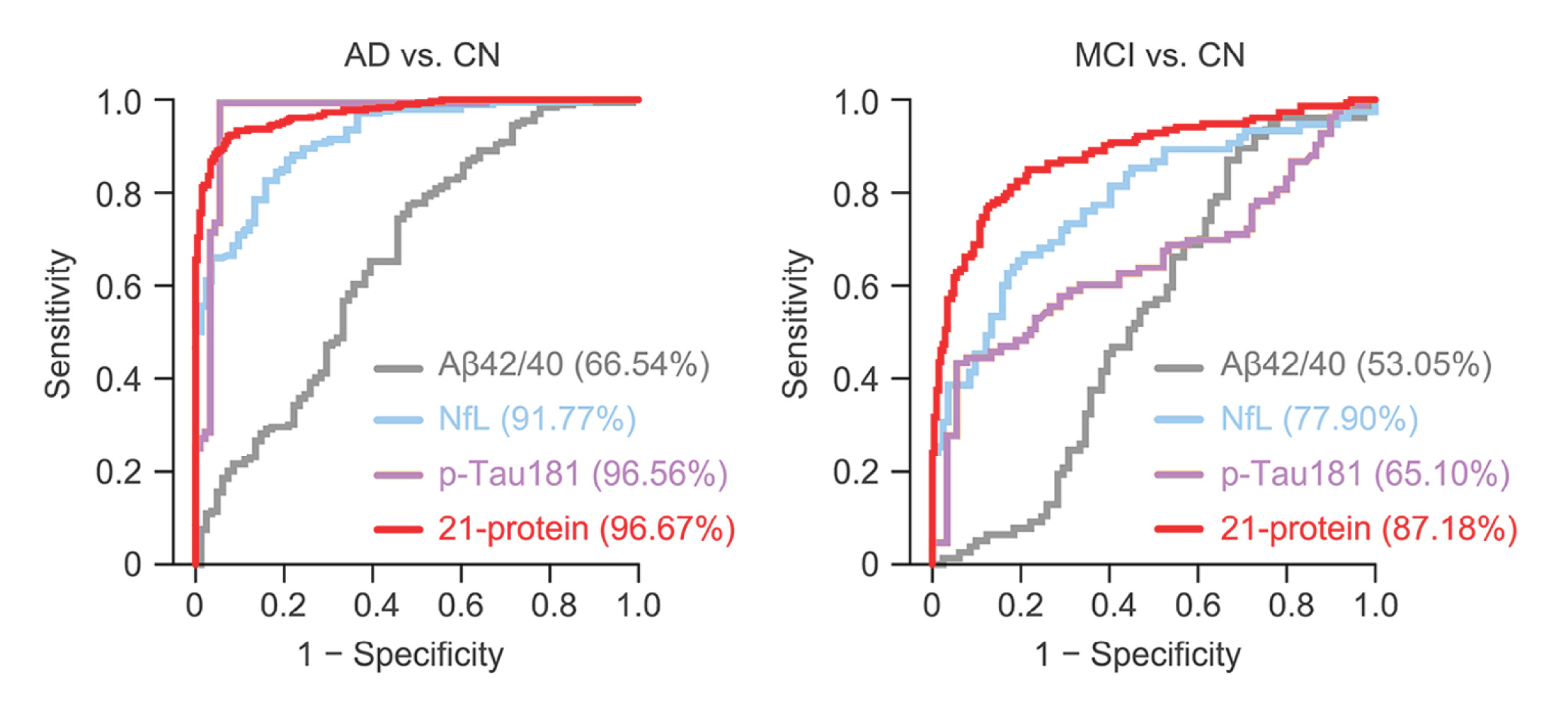
An international research collaboration led by Professor Nancy IP, has made a significant breakthrough in Alzheimer’s disease (AD) diagnosis and management. The team has developed a cutting-edge blood test for the early detection of AD and mild cognitive impairment (MCI) with remarkable accuracy rates of over 96% and 87% respectively. This blood test is applicable across diverse ethnic populations, providing a global solution to the diagnosis and management of AD.
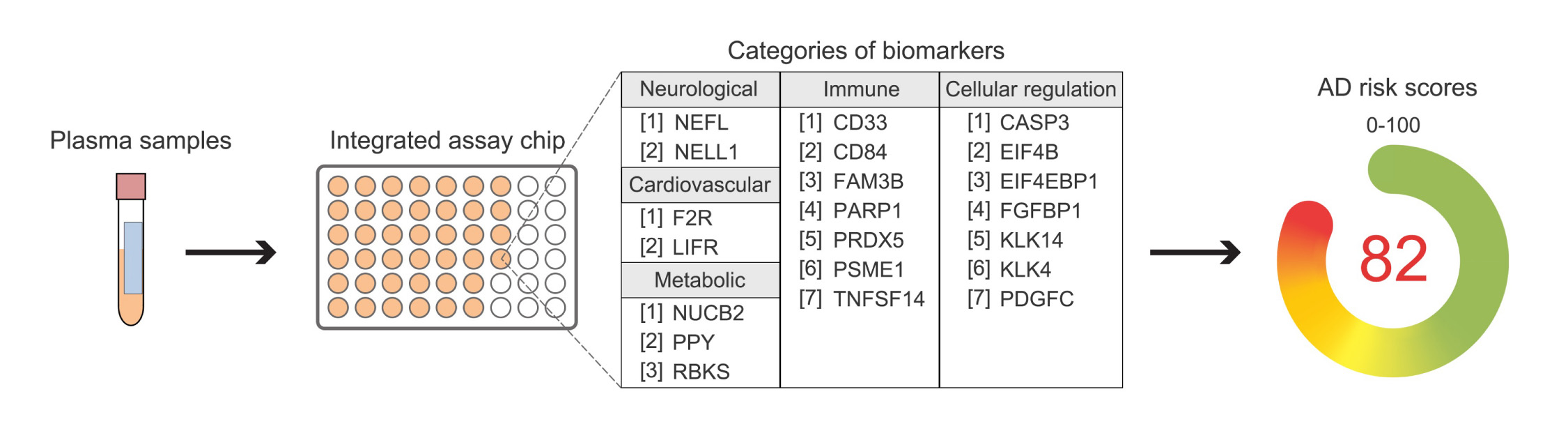
AD is a neurodegenerative disease that affects over 50 million people worldwide. The accumulation of toxic amyloid beta (Aβ) in the brain is a major hallmark of the disease, leading to the loss of brain cells and cognitive decline. The recently approved AD drug Lecanemab targets elevated Aβ in the brain, offering new hope for treatment. However, the majority of individuals with AD and MCI go undiagnosed and untreated due to the challenges of early diagnosis. Currently, elevated Aβ can only be measured through costly brain imaging or invasive procedures, and symptoms typically appear late in the disease’s progression.
The HKUST-led research team has developed a simple blood test that accurately identifies individuals with MCI and mild AD, while also detecting elevated Aβ in the brain. The test measures the levels of 21 proteins in multiple biological pathways, providing a comprehensive profile of AD for each individual. This allows for more accurate classification of AD and MCI, as well as close monitoring of disease progression. The test has demonstrated robust performance in distinguishing individuals with AD and MCI from cognitively normal people in a multinational study involving individuals of Chinese and European descent.
The HKUST blood test represents a significant advancement in AD diagnosis and precision treatment. It offers a less invasive and more effective diagnostic method and has the potential to revolutionize the field of AD management. The test can also be used to screen individuals for suitable drug treatments in clinical studies and monitor disease progression and drug responses. Additionally, it may facilitate the development of personalized treatments by uncovering the molecular underpinnings of AD that vary between individuals and ethnicities.
The research collaboration involved University College London and the Barcelona βeta Brain Research Center, as well as clinicians from local and overseas hospitals.
The findings have been published in Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, and the technology has been licensed to a start-up company of HKUST, Cognitact Limited, to provide testing services to the public.
Journal Reference:
Jiang Y, Zhou X, Ip FC, Chan P, Chen Y, Lai NCH, Cheung K, Lo RMN, Tong EPS, Wong BWY, Chan ALT, Mok VCT, Kwok TCY, Mok KY, Hardy J, Zetterberg H, Fu AKY, Ip NY. Large-scale plasma proteomic profiling identifies a high-performance biomarker panel for Alzheimer’s disease screening and staging. Alzheimers Dement. 2022 Jan;18(1):88-102. doi: 10.1002/alz.12369.
For further information please visit:
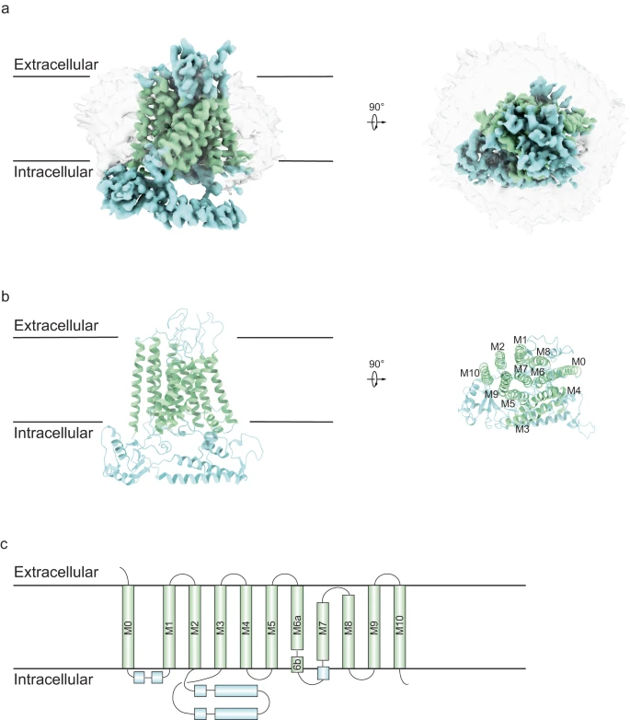
A study led by Professor Shanyu Dang and his research team at HKUST is shedding light on TMEM63 proteins. These proteins are calcium-permeable channels in animals that are primarily activated by hypo-osmolality, and they play crucial roles in various physiological functions. Deficiencies in these channels have been associated with several diseases, including hearing loss. However, their structures and physiological roles have remained elusive until now.
To investigate these proteins, the team utilized cryo-electron microscopy (cryo-EM) to examine the structure of TMEM63C in mouse and compared it to other TMEM63 proteins as well as their plant orthologues known as OSCAs. Notably, they discovered significant differences in structure among these proteins.
Furthermore, the researchers conducted experiments to understand the functioning of TMEM63 proteins. They identified specific regions of the protein that are essential for its activity and elucidated the critical roles of the coupling between TM0 and TM6 in channel activity. Additionally, they observed that TMEM63C acts as a single unit, while TMEM63B can exist as both a single unit and a pair. This suggests that TMEM63 proteins can regulate their function through their dimerization.
Understanding TMEM63 proteins is crucial because they are involved in how cells perceive and respond to mechanical forces. These forces are vital for cellular adaptation and survival in their environment.
The findings of this study provide valuable insights into the structure and function of TMEM63 proteins, opening up possibilities for the development of new treatments for diseases such as hearing loss.
Journal Reference:
Qin, Y., Yu, D., Wu, D. et al. Cryo-EM structure of TMEM63C suggests it functions as a monomer. Nat Commun 14, 7265 (2023). https://doi.org/10.1038/s41467-023-42956-2
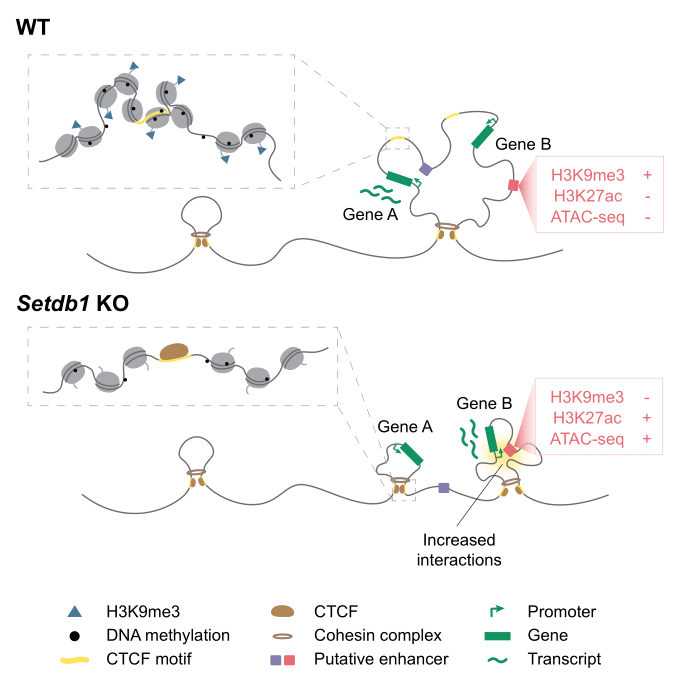
This discovery has important implications for understanding how genes are turned on and off. The researchers also found that when SETDB1 and H3K9me3 are lost, it leads to changes in how our DNA is organized, affecting gene activity and repetitive elements in our genome. This research provides valuable insights into the mechanisms behind gene regulation and opens up new possibilities for developing targeted therapies to manipulate gene activity, which could be particularly relevant for diseases like cancer.
Journal Reference:
Tam, P.L.F., Cheung, M.F., Chan, L.Y. et al. Cell-type differential targeting of SETDB1 prevents aberrant CTCF binding, chromatin looping, and cis-regulatory interactions. Nat Commun 15, 15 (2024). https://doi.org/10.1038/s41467-023-44578-0
An interview with Prof. Danny Leung, associate professor of LIFS on pre-eclampsia (a complex condition characterized by high blood pressure and organ damage in pregnant women) involving impaired placental development and the release of anti-angiogenic factors, and its relationship with COVID-19 was featured in the Chinese edition of Scientific America.
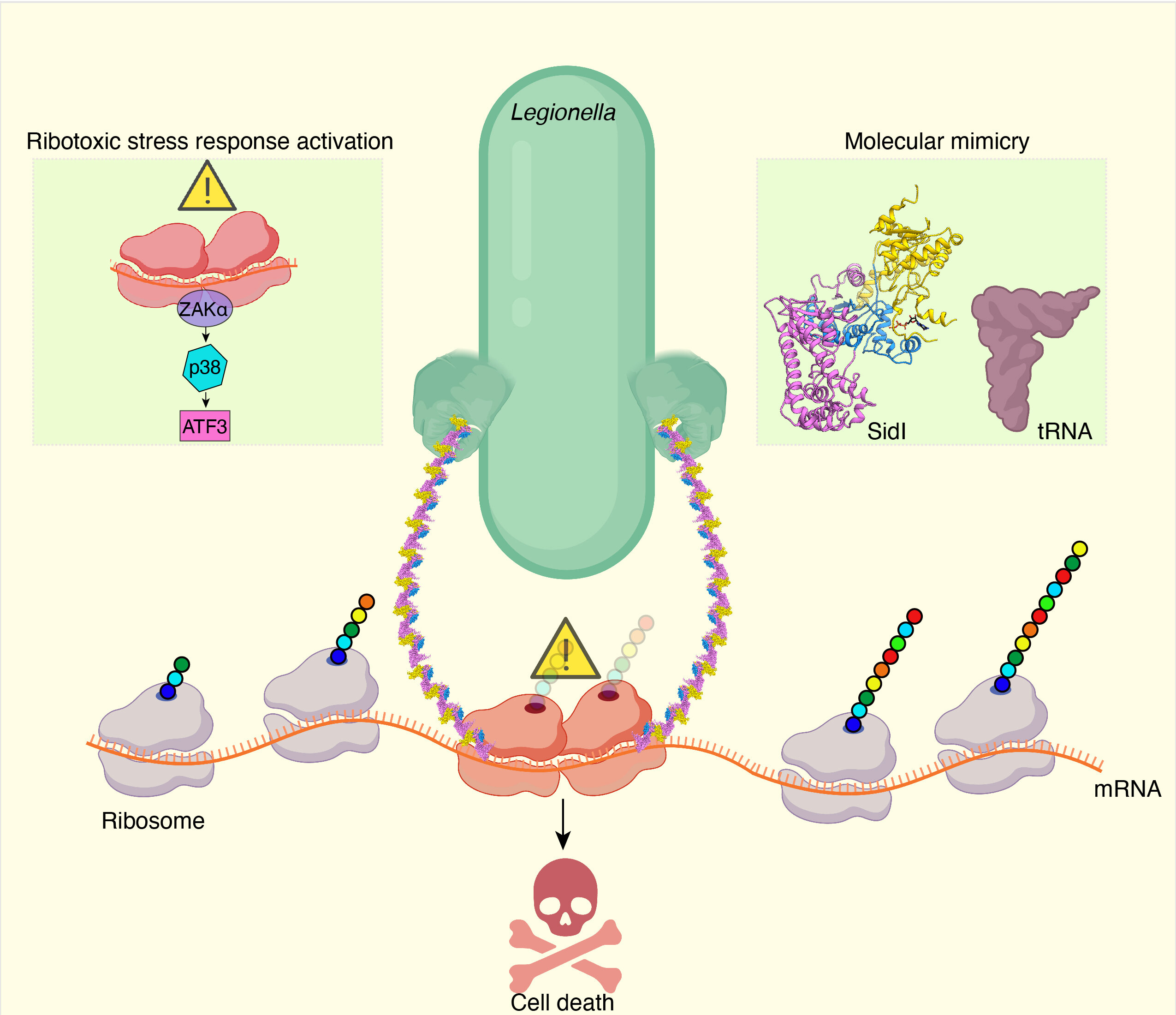
Legionella pneumophila, the bacterium responsible for Legionnaires’ disease, employs a unique strategy to disrupt protein synthesis in host cells. One of its secreted proteins, SidI, functions as a transfer-RNA mimic, directly binding to and modifying the ribosome.
Through cryo-electron microscopy the structure of SidI was revealed, with its tRNA-like N-terminal domain and glycosyl transferase C-terminal domain. SidI’s tRNA mimicry and enzymatic activity effectively block protein synthesis akin to the potent toxin ricin. The translational pauses induced by SidI trigger a stress response similar to ribosome collisions, activating stress kinases and leading to the accumulation of ATF3, a transcription factor promoting cell death. This mechanism unveils how Legionella manipulates a ribosome-to-nuclear signaling pathway. The study was carried out by Prof. Lan Wang from the Division of Life Science at HKUST and Dr. Advait Subramanian from Altos Labs Inc. in California. Their research on SidI sheds light on the use of molecular mimicry by pathogens to hijack critical cellular processes, providing fundamental insights into host cell function and stress response pathways.
Journal Reference:
Subramanian, A., Wang, L., Moss, T. et al. A Legionella toxin exhibits tRNA mimicry and glycosyl transferase activity to target the translation machinery and trigger a ribotoxic stress response. Nat Cell Biol 25, 1600–1615 (2023). https://doi.org/10.1038/s41556-023-01248-z
Source: Phys.org News Dec 5 2023


Dr. WANG Lan (HKUST)
Assistant Professor, Division of Life Science,
The Hong Kong University of Science and Technology
One of our LIFS faculty members, Dr. Wang Lan, Assistant Professor of the Division of Life Science, received the Croucher Innovation Award, 2023. Prof. SUN Dong, the Secretary for Innovation, Technology and Industry of the Hong Kong Special Administrative Region was the Guest of Honor at the ceremony.
Dr. Wang is interested in understanding the molecular mechanisms that determine the health of the mitochondria. Mitochondrial dysfunction underlies many human diseases. Additionally, Dr. Wang is also interested in discovering new mechanisms that regulate protein translation and how pathogens could exploit these mechanisms to manipulate the host cell for their own advantage.
Dr. Wang joined HKUST in 2022. She studied chemistry as an undergraduate at Tsinghua University and obtained her PhD at Harvard University. She subsequently worked as a postdoctoral fellow at UCSF.
Croucher Innovation Awards
Established in 2012, the Croucher Innovation Awards aim to identify a small number of exceptionally talented scientists working at an internationally competitive level and to offer substantial support to these “rising stars” at a formative stage in their careers. The scheme is designed to enable recipients to pursue their own scientific, intellectual and professional inclinations, to advance their expertise, to engage in bold new work, and to contribute to the development of education and research in Hong Kong. Each award carries a value of up to HK$5 million over five years.
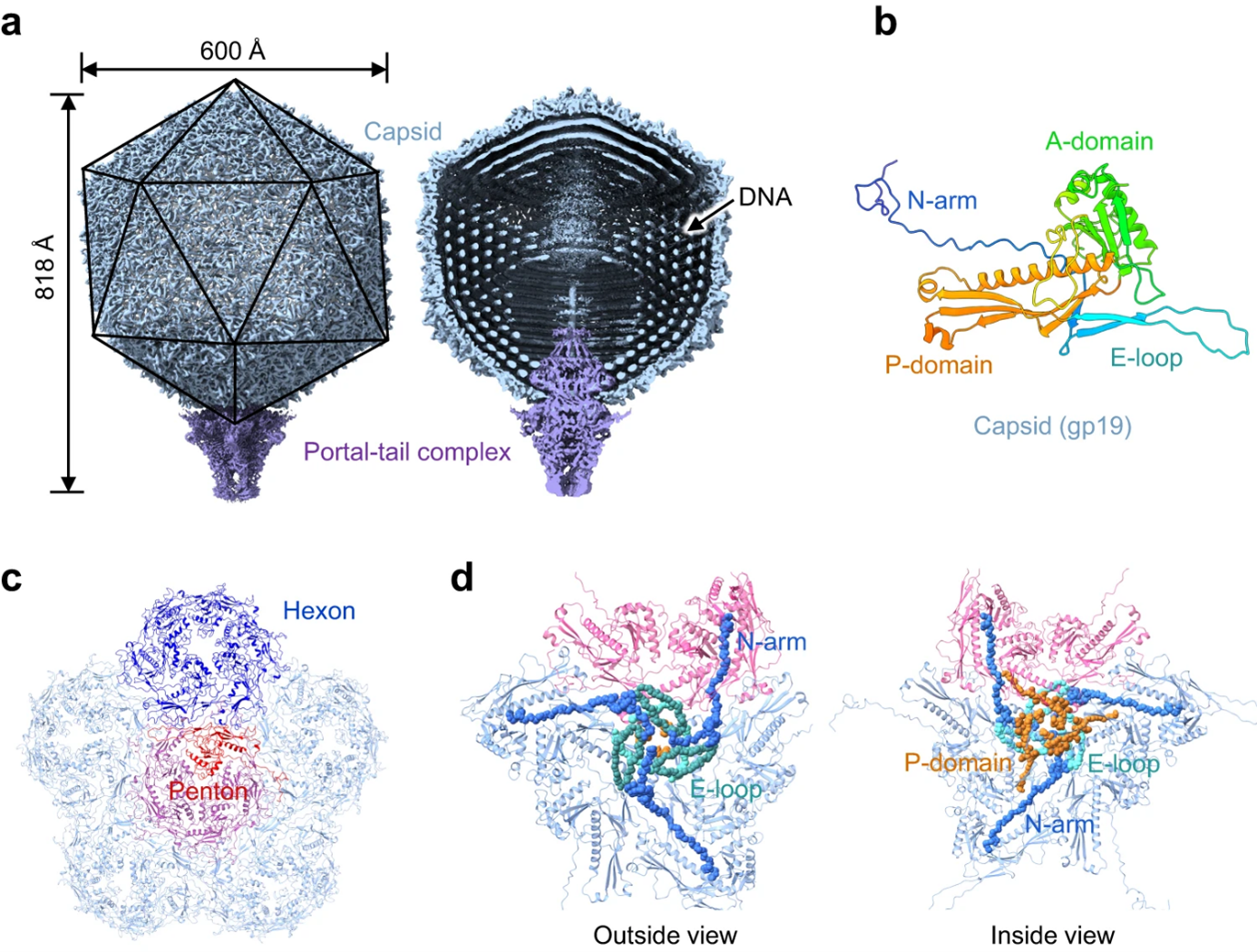
Cyanobacteria are crucial for Earth’s ecosystems as they produce oxygen and serve as a food and fuel source. Infections by cyanophages can alter bacterial behavior, affecting their energy production from sunlight and causing significant carbon loss annually. Understanding the viruses’ mechanisms and their impact on bacteria, oceans, and climate is essential.
Moreover, insights into their infection processes can aid in the development of virus-based strategies to control harmful cyanobacteria in freshwater, benefiting agriculture and ensuring clean drinking water. The team’s findings were published in the journal Nature Communications, and future research aims to further explore the virus’s infection process and resolve unresolved parts of its structure relating to host recognition and infection.
Journal Reference:
Cai, L., Liu, H., Zhang, W. et al. Cryo-EM structure of cyanophage P-SCSP1u offers insights into DNA gating and evolution of T7-like viruses. Nat Commun 14, 6438 (2023). https://doi.org/10.1038/s41467-023-42258-7
Read More
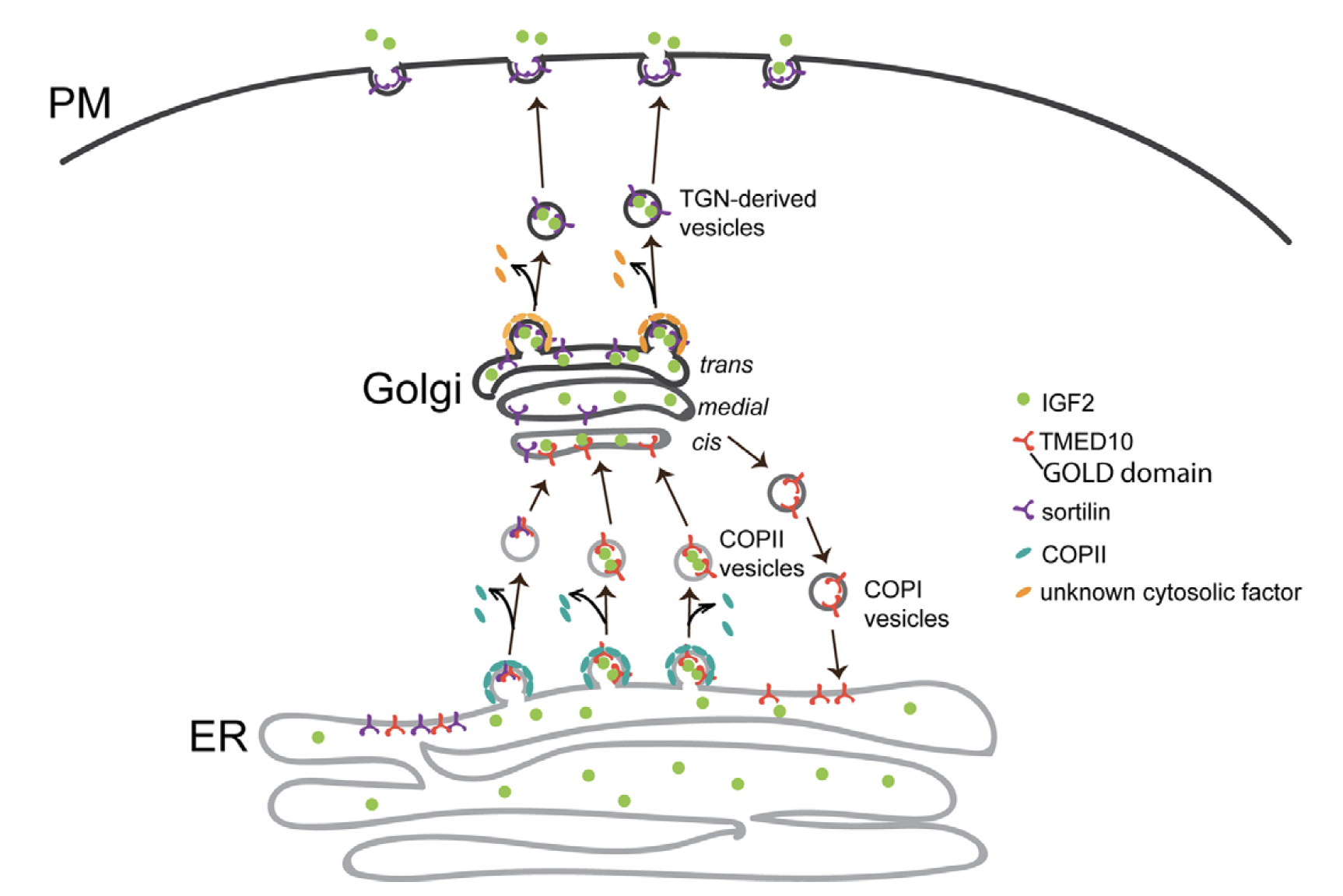
Journal Reference:
Tiantian Li,, Feng Yang, Youshan Heng, Shaopu Zhou, Gang Wang, Jianying Wang, Jinhui Wang, Xianwei Chen, Zhong- Ping Yao, Zhenguo Wu, and Yusong Guo. TMED10 mediates the trafficking of insulin- like growth factor 2 along the secretory pathway for myoblast differentiation. PNAS 2023 Vol. 120 No. 46 e2215285120
https://doi.org/10.1073/pnas.2215285120
HKUST President Prof. Nancy IP (center, front row), Research Professor Prof. Amy FU (second right, front row), a co-first author of this research paper Mr. WU Wei (second left, front row), and Research Assistant Professor Prof. WONG Hiu Yi (first left, front row) with other members of the HKUST Division of Life Science research team.

Dr. LAU Shun-Fat, a co-first author of this research paper and former Postdoctoral Fellow at HKUST Division of Life Science, is now a Postdoctoral Associate at the Yale University School of Medicine.
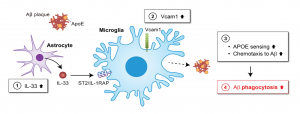
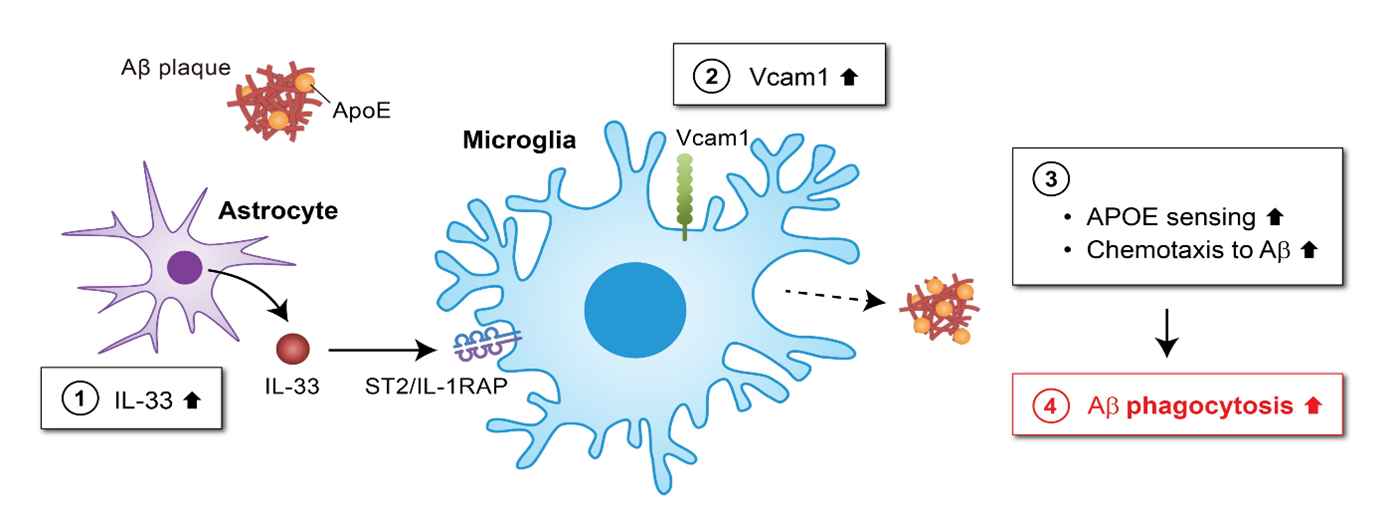
The diagram illustrates the VCAM1-APOE signalling pathway as a potential therapeutic target for Alzheimer’s disease. Interleukin-33 (IL-33) (1) increases VCAM1 expression in microglia (2), which stimulates the attraction of microglia to APOE-associated amyloid-β (Aβ) deposition (3), leading to the clearance of Aβ in the brain (4).
Journal Reference:
Shun-Fat Lau, Wei Wu, Hiu Yi Wong, Li Ouyang, Yi Qiao, Jiahui Xu, Jessica Hiu-Yan Lau, Carlton Wong, Yuanbing Jiang, David M Holtzman, Amy K Y Fu, Nancy Y Ip. The VCAM1-ApoE pathway directs microglial chemotaxis and alleviates Alzheimer’s disease pathology.
Nat Aging 2023 Oct;3(10):1219-1236. doi: 10.1038/s43587-023-00491-1.
Read More