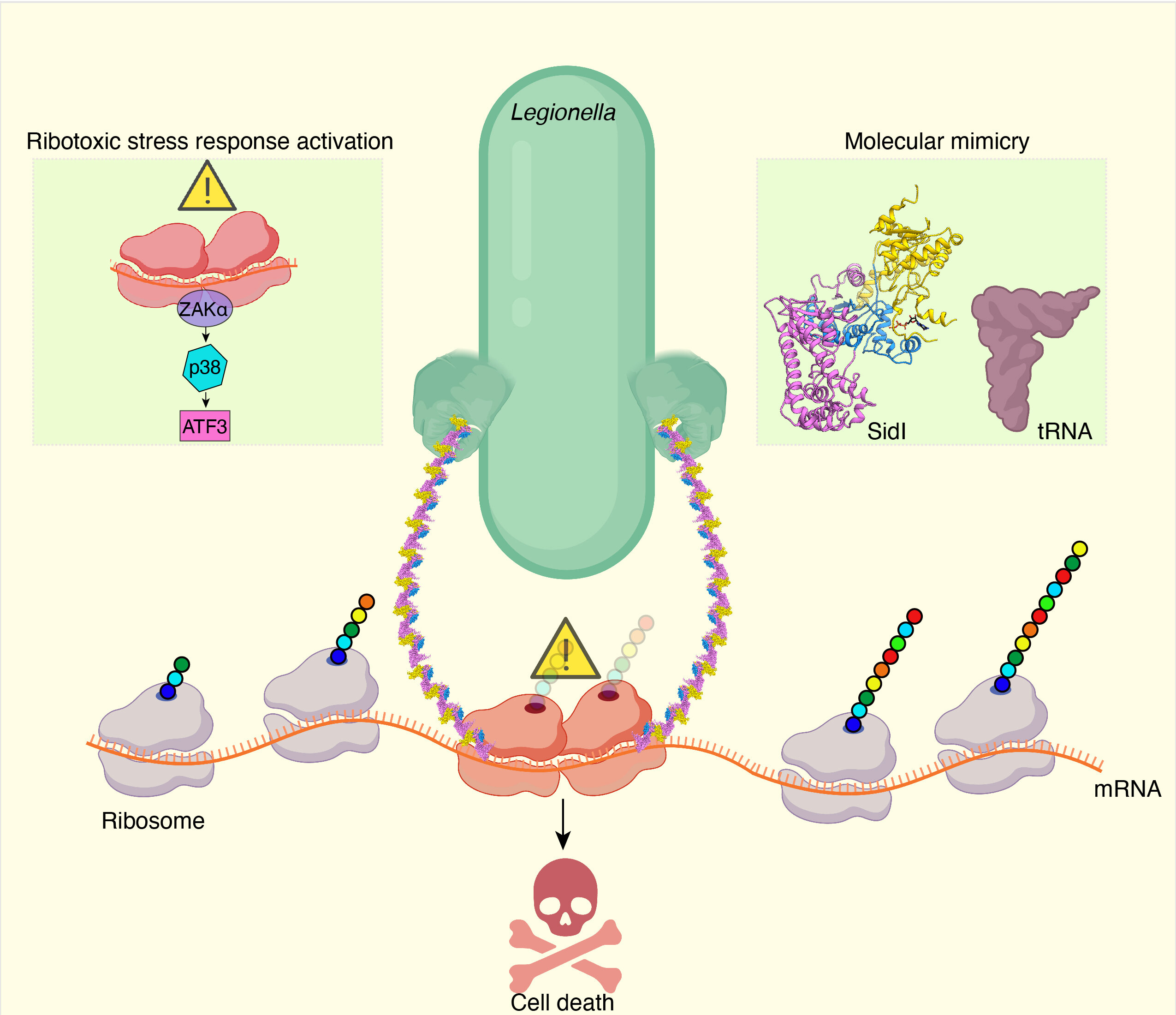
Legionella pneumophila, the bacterium responsible for Legionnaires’ disease, employs a unique strategy to disrupt protein synthesis in host cells. One of its secreted proteins, SidI, functions as a transfer-RNA mimic, directly binding to and modifying the ribosome.
Through cryo-electron microscopy the structure of SidI was revealed, with its tRNA-like N-terminal domain and glycosyl transferase C-terminal domain. SidI’s tRNA mimicry and enzymatic activity effectively block protein synthesis akin to the potent toxin ricin. The translational pauses induced by SidI trigger a stress response similar to ribosome collisions, activating stress kinases and leading to the accumulation of ATF3, a transcription factor promoting cell death. This mechanism unveils how Legionella manipulates a ribosome-to-nuclear signaling pathway. The study was carried out by Prof. Lan Wang from the Division of Life Science at HKUST and Dr. Advait Subramanian from Altos Labs Inc. in California. Their research on SidI sheds light on the use of molecular mimicry by pathogens to hijack critical cellular processes, providing fundamental insights into host cell function and stress response pathways.
Journal Reference:
Subramanian, A., Wang, L., Moss, T. et al. A Legionella toxin exhibits tRNA mimicry and glycosyl transferase activity to target the translation machinery and trigger a ribotoxic stress response. Nat Cell Biol 25, 1600–1615 (2023). https://doi.org/10.1038/s41556-023-01248-z
Source: Phys.org News Dec 5 2023